Contemporary phytotherapy leverages scientific advancements to refine traditional botanical treatments. This approach incorporates rigorous research methodologies, standardized extraction processes, and quality control measures to enhance the efficacy and safety of plant-derived products. For example, specific compounds from plants are isolated, tested in clinical trials, and formulated into precise dosages for targeted health applications.
The significance of evidence-based botanical medicine lies in its potential to offer complementary or alternative options for managing various health conditions. Historically, plant-based therapies have been integral to healing practices across cultures. Current research aims to validate these traditional uses while optimizing therapeutic outcomes and minimizing potential risks. This approach often results in formulations with quantified active ingredients, ensuring consistent and predictable effects.
The subsequent sections will delve into the specific applications of these therapies, exploring their role in supporting overall wellness, addressing common ailments, and potentially contributing to preventative healthcare strategies. Furthermore, it will address regulatory frameworks, quality assurance protocols, and considerations for informed patient decision-making within this evolving field.
Guidance on Leveraging Botanical Therapies
This section provides key considerations for individuals seeking to integrate scientifically-validated botanical approaches into their health regimen. It emphasizes informed decision-making and responsible utilization.
Tip 1: Prioritize Products with Standardized Extracts: Opt for formulations that quantify the levels of active compounds. This ensures batch-to-batch consistency and predictable therapeutic effects. For example, select a milk thistle supplement standardized to a specific percentage of silymarin.
Tip 2: Consult with Qualified Healthcare Professionals: Engage with physicians, pharmacists, or registered herbalists knowledgeable in botanical medicine. They can assess potential interactions with existing medications and provide personalized recommendations based on individual health needs.
Tip 3: Research Reputable Brands: Investigate the manufacturing practices, quality control procedures, and independent testing certifications of product manufacturers. Third-party verification, such as USP or NSF seals, indicates adherence to established quality standards.
Tip 4: Be Aware of Potential Interactions: Certain botanicals can interact with pharmaceutical drugs, altering their efficacy or increasing the risk of adverse effects. For instance, St. John’s Wort is known to interact with several medications, including antidepressants and oral contraceptives.
Tip 5: Start with Low Dosages: When initiating any new botanical regimen, begin with the lowest recommended dose to assess individual tolerance and minimize potential side effects. Gradually increase the dosage as needed, under the guidance of a healthcare professional.
Tip 6: Monitor for Adverse Reactions: Pay close attention to any unusual symptoms or changes in health status after starting a botanical product. Discontinue use and seek medical advice if adverse reactions occur, such as allergic reactions or gastrointestinal distress.
Tip 7: Understand the Evidence Base: Review the scientific literature and clinical trial data supporting the use of specific botanicals for particular health conditions. Recognize that evidence may vary, and some uses may be based primarily on traditional practices.
Employing these strategies promotes a safe and effective approach to botanical therapies, allowing individuals to harness their potential benefits while minimizing potential risks.
The subsequent section will explore the regulatory landscape surrounding botanical products, further emphasizing the importance of informed consumer choices and adherence to established quality standards.
1. Efficacy
Efficacy, in the context of botanical therapeutics, refers to the capacity of a preparation to produce a desired therapeutic effect in controlled clinical trials. The establishment of efficacy is paramount for integrating these remedies into modern healthcare practices. Without demonstrable effectiveness, any purported benefits remain speculative, potentially misleading patients and hindering optimal treatment strategies. For instance, the clinical efficacy of St. John’s Wort for mild to moderate depression has been investigated in numerous randomized controlled trials, providing evidence-based support for its use in specific cases. In contrast, other traditional herbal applications lack sufficient scientific validation, necessitating caution and further research.
The process of evaluating efficacy involves rigorous methodologies, including placebo-controlled studies and comparisons with conventional treatments. These studies assess various outcome measures, such as symptom reduction, disease progression, and quality of life improvements. The results are then subjected to statistical analysis to determine the significance of the observed effects. An example is the use of curcumin, a compound derived from turmeric, in managing osteoarthritis. Clinical trials have explored its impact on pain reduction and joint function, contributing to a growing body of evidence supporting its potential therapeutic role. However, it’s crucial to acknowledge that the efficacy of botanical remedies can vary depending on factors such as plant species, extraction methods, dosage, and individual patient characteristics.
Ultimately, the assessment of efficacy determines the legitimacy and potential value of any botanical intervention. Challenges remain in standardizing research methodologies and accounting for the complex interactions between plant constituents. However, continued investment in rigorous scientific investigation is essential for translating traditional knowledge into evidence-based healthcare solutions. This pursuit of verified efficacy empowers practitioners to make informed decisions and enables patients to access reliable and effective treatment options.
2. Safety
The integration of plant-derived products into modern healthcare necessitates a stringent focus on safety, moving beyond traditional use to encompass evidence-based risk assessment and mitigation. Adverse effects, while potentially infrequent, can arise from inherent toxicity, allergic reactions, interactions with pharmaceutical medications, or contamination during cultivation, processing, or manufacturing. An example of a safety concern involves pyrrolizidine alkaloids (PAs) found in certain plant species like comfrey. These compounds can cause liver damage with prolonged or excessive consumption, highlighting the importance of identifying and avoiding such plants.
Modern herbal practices emphasize several factors to ensure safety. Proper identification of the plant species is critical, as misidentification can lead to the use of toxic substitutes. Standardized extraction and manufacturing processes are essential to minimize contamination and ensure consistent potency. Furthermore, thorough assessment of potential drug interactions is vital, as certain herbs can alter the metabolism or effects of pharmaceutical medications. For example, St. John’s Wort, a widely used herb for depression, can interact with numerous drugs, including antidepressants, oral contraceptives, and anticoagulants. Therefore, healthcare professionals must carefully evaluate patients’ medication profiles before recommending any botanical remedy.
Ultimately, ensuring the safety of plant-based interventions requires a multi-faceted approach encompassing rigorous quality control, thorough risk assessment, and informed clinical decision-making. While botanical products may offer therapeutic benefits, their potential risks must be carefully considered and managed to protect patient well-being. Ongoing research and pharmacovigilance programs play a crucial role in identifying and addressing emerging safety concerns associated with these therapies.
3. Standardization
Standardization is an indispensable aspect of integrating plant-derived products into contemporary healthcare practices. It addresses the inherent variability in plant composition, ensuring consistent potency and predictable therapeutic effects. This process is pivotal for establishing reliability and reproducibility, essential for scientific validation and clinical application.
- Chemical Profiling
Chemical profiling involves identifying and quantifying key bioactive compounds within a botanical extract. Techniques such as chromatography and mass spectrometry are employed to create a detailed fingerprint of the plant’s chemical constituents. For example, a standardized extract of Echinacea may specify the concentrations of alkamides and cichoric acid, ensuring consistent immunological activity. The absence of rigorous chemical profiling can lead to batch-to-batch variations, compromising therapeutic efficacy and potentially posing safety risks.
- Extraction Methods
The extraction method significantly influences the composition and yield of bioactive compounds. Standardized extraction protocols, such as supercritical fluid extraction or solvent extraction with controlled parameters (temperature, pressure, solvent ratio), are crucial for minimizing variability. For instance, a standardized Ginkgo biloba extract utilizes a specific solvent ratio to selectively extract ginkgo flavone glycosides and terpene lactones, while minimizing the presence of unwanted compounds. Inconsistent extraction processes can result in variations in the concentration of active constituents, affecting the therapeutic outcome.
- Dosage Forms
Standardization extends to the formulation of dosage forms, ensuring consistent delivery of the active compounds. This involves precise manufacturing processes and quality control measures to guarantee uniform tablet weight, capsule fill, or liquid concentration. An example is a standardized valerian root extract encapsulated with a specified amount of valerenic acid, ensuring consistent sedative effects. Variations in dosage form manufacturing can lead to inconsistencies in the amount of active ingredient delivered, potentially affecting therapeutic efficacy.
- Quality Control and Assurance
Robust quality control measures are essential throughout the standardization process, encompassing raw material sourcing, manufacturing, and finished product testing. These measures include identity testing, purity analysis, and potency assays to ensure adherence to established specifications. Third-party certification programs, such as USP or NSF, provide independent verification of product quality and standardization. Without stringent quality control, botanical products may contain contaminants, adulterants, or inaccurate amounts of active constituents, compromising safety and efficacy.
These facets of standardization collectively contribute to the reliability and predictability of botanical therapies. By implementing rigorous chemical profiling, controlled extraction methods, consistent dosage forms, and robust quality control measures, manufacturers can produce botanical products that meet established quality standards. This is a cornerstone for integrating them into evidence-based healthcare, facilitating informed decision-making by healthcare professionals and ensuring consistent therapeutic outcomes for patients. This holistic approach also aids in bridging the gap between traditional practices and modern medicine, fostering trust and acceptance in the use of these natural remedies.
4. Regulation
The regulatory landscape surrounding plant-derived therapeutic agents directly influences their availability, quality, and safe integration into healthcare systems. Differing global regulations create variable standards, impacting both consumers and healthcare professionals seeking reliable therapeutic options.
- Market Access and Licensing
Regulatory bodies, such as the FDA in the United States and the EMA in Europe, determine the requirements for market access. These requirements can range from minimal registration for traditional use products to stringent clinical trial data for novel botanical drugs. For example, in Germany, certain herbal medicines with established traditional use are licensed based on monographs published by the Commission E, while in the US, many herbal products are marketed as dietary supplements, subject to less rigorous pre-market scrutiny. The absence of consistent global standards creates challenges for international trade and consumer confidence.
- Quality Control and Manufacturing Standards
Good Manufacturing Practices (GMP) guidelines, enforced by regulatory agencies, dictate the manufacturing processes, quality control procedures, and documentation requirements for botanical products. GMP compliance aims to ensure product consistency, purity, and safety. For instance, GMP regulations mandate that manufacturers implement measures to prevent contamination, accurately identify plant species, and verify the potency of active constituents. Failure to adhere to GMP standards can result in product recalls, fines, and legal action.
- Health Claims and Labeling Requirements
Regulations govern the types of health claims that can be made on product labels and in marketing materials. These regulations aim to prevent misleading or unsubstantiated claims. For example, in many jurisdictions, botanical products cannot be marketed with claims to diagnose, treat, or cure serious diseases without prior approval from regulatory authorities. Clear and accurate labeling, including ingredient lists, dosage instructions, and potential warnings, is also essential for informed consumer decision-making.
- Pharmacovigilance and Adverse Event Reporting
Regulatory systems establish pharmacovigilance programs to monitor the safety of botanical products after they have been released onto the market. Healthcare professionals and consumers are encouraged to report adverse events associated with these products, allowing regulatory agencies to identify potential safety signals and take appropriate action. For instance, if a cluster of liver toxicity cases is linked to a particular herbal product, the regulatory agency may issue a warning or recall the product from the market. The effectiveness of pharmacovigilance systems depends on accurate and timely reporting of adverse events.
These regulatory facets shape the development, manufacturing, and distribution of contemporary botanical treatments. While regulations seek to ensure product quality and safety, variations across jurisdictions create complexities for manufacturers and consumers alike. Harmonization of regulatory standards and enhanced collaboration among global regulatory agencies are essential for fostering a transparent and reliable marketplace for plant-based health products.
5. Research
Scientific inquiry forms the bedrock of legitimizing contemporary botanical therapies. Rigorous investigation elucidates the mechanisms of action, validates traditional uses, and identifies potential risks associated with plant-derived compounds. The absence of comprehensive research undermines the integration of such remedies into evidence-based medical practice. For instance, the investigation of artemisinin, derived from Artemisia annua, into a potent anti-malarial drug showcases the impact of scientific research on transforming a traditional herbal remedy into a life-saving treatment. Conversely, reliance on anecdotal evidence without empirical validation can perpetuate the use of ineffective or harmful botanical interventions.
The multifaceted approach to botanical research encompasses various methodologies, including in vitro studies, animal models, and human clinical trials. In vitro studies, utilizing cell cultures, are instrumental in dissecting the molecular mechanisms of action and identifying potential drug interactions. Animal models provide valuable insights into the pharmacokinetics, pharmacodynamics, and toxicity of plant extracts. Human clinical trials, including randomized controlled trials (RCTs), are essential for evaluating the efficacy and safety of botanical treatments in diverse patient populations. An example is the extensive research on turmeric and its active constituent, curcumin, which have been explored for their anti-inflammatory and antioxidant properties in various clinical trials targeting conditions like arthritis and inflammatory bowel disease. The rigor and reproducibility of these studies determine the validity and generalizability of the findings.
In conclusion, research serves as the linchpin for rationalizing and optimizing the utilization of contemporary botanical therapeutics. It differentiates evidence-based interventions from unsubstantiated claims, ensuring patient safety and promoting informed decision-making by healthcare professionals. Continued investment in rigorous scientific inquiry is imperative for unlocking the full therapeutic potential of the natural world and integrating plant-derived compounds into mainstream medical practice. The complexities inherent in botanical research, such as standardization of plant materials and identification of active constituents, necessitate interdisciplinary collaboration among botanists, chemists, pharmacologists, and clinicians to address these challenges effectively. The results translate directly to better-informed practices and improved patient outcomes.
6. Integration
Integration, in the context of contemporary botanical therapeutics, signifies the seamless and evidence-based incorporation of plant-derived treatments into conventional healthcare systems. This process necessitates a collaborative approach among healthcare practitioners, researchers, and regulatory bodies, emphasizing patient-centered care and promoting informed decision-making.
- Interprofessional Collaboration
Effective integration mandates communication and collaboration among medical doctors, pharmacists, nurses, and herbalists. This collaborative model fosters a holistic approach to patient care, combining the strengths of conventional medicine with the potential benefits of botanical therapies. For example, an oncologist may collaborate with a qualified herbalist to mitigate the side effects of chemotherapy using scientifically validated botanical interventions. Open communication ensures that potential drug interactions are identified and managed effectively, optimizing patient outcomes. The interprofessional approach is not merely additive but synergistic, enhancing the efficacy of both conventional and botanical treatments through comprehensive patient assessment and monitoring.
- Evidence-Based Practice
The integration of botanical therapies must be grounded in scientific evidence. This entails prioritizing treatments with established efficacy and safety profiles, supported by rigorous clinical trials and systematic reviews. For instance, the use of ginger for managing nausea during pregnancy is supported by numerous studies demonstrating its effectiveness and safety. Conversely, the promotion of botanical treatments with unsubstantiated claims undermines the credibility of botanical medicine and may pose risks to patient health. Evidence-based practice necessitates continuous evaluation of emerging research and critical appraisal of traditional uses, ensuring that botanical therapies are utilized responsibly and ethically.
- Patient Education and Empowerment
Integration necessitates providing patients with comprehensive and unbiased information about botanical therapies, enabling them to make informed decisions about their healthcare. This includes educating patients about the potential benefits, risks, and interactions of plant-derived treatments, as well as the importance of consulting with qualified healthcare professionals. For example, a patient considering St. John’s Wort for depression should be informed about its potential interactions with antidepressant medications and the importance of monitoring for adverse effects. Empowering patients with knowledge fosters a collaborative relationship with their healthcare providers and promotes adherence to treatment plans.
- Standardized Protocols and Guidelines
The implementation of standardized protocols and clinical guidelines is essential for ensuring consistent and safe utilization of botanical therapies within healthcare settings. These guidelines should specify appropriate indications, dosages, contraindications, and monitoring parameters for various botanical treatments. For instance, a hospital may develop a protocol for the use of chamomile tea to promote sleep in patients with insomnia, specifying the type of chamomile, brewing instructions, and potential adverse effects. Standardized protocols enhance the quality and consistency of care, reducing the risk of errors and adverse events.
These facets of integration collectively represent a paradigm shift towards a more holistic and patient-centered approach to healthcare. By fostering interprofessional collaboration, prioritizing evidence-based practice, empowering patients with knowledge, and implementing standardized protocols, healthcare systems can effectively harness the potential benefits of botanical therapeutics while mitigating potential risks. Further research and ongoing dialogue are essential for refining integration strategies and optimizing the utilization of plant-derived treatments in the 21st century.
Frequently Asked Questions
This section addresses common inquiries and misconceptions surrounding the application of plant-derived compounds in contemporary healthcare. The information provided aims to offer clarity and promote a more informed understanding.
Question 1: Are “modern herbal remedies” safe for everyone to use?
Safety profiles of phytotherapeutic agents vary considerably. While some botanicals possess a long history of safe use, others may carry risks of adverse effects or interactions with pharmaceutical medications. Consultation with a qualified healthcare professional is crucial to assess individual suitability and mitigate potential risks.
Question 2: How are “modern herbal remedies” different from traditional herbal medicine?
Contemporary applications emphasize scientific validation, standardization, and quality control. Traditional herbalism often relies on anecdotal evidence and may lack precise dosage guidelines. “Modern herbal remedies” aim to integrate evidence-based practices with time-honored traditions, utilizing advanced extraction and analytical techniques.
Question 3: Can “modern herbal remedies” replace conventional medical treatments?
These therapies are typically not intended as replacements for conventional medical care, particularly for serious health conditions. They may serve as complementary or adjunctive treatments, potentially enhancing the efficacy of conventional therapies or mitigating side effects. Consultation with a physician is essential before altering or discontinuing prescribed medications.
Question 4: How can the quality of “modern herbal remedies” be assessed?
Look for products manufactured according to Good Manufacturing Practices (GMP) and certified by independent third-party organizations (e.g., USP, NSF). Scrutinize labels for standardized extracts, specifying the concentration of active compounds. Research the manufacturer’s reputation and commitment to quality control.
Question 5: Are there specific regulations governing “modern herbal remedies”?
Regulatory frameworks vary across countries. In some regions, botanical products are regulated as dietary supplements, subject to less stringent requirements than pharmaceutical drugs. Other jurisdictions have specific licensing requirements for herbal medicines with established traditional use. Consumers should be aware of the regulatory status of botanical products in their respective regions.
Question 6: Where can reliable information about “modern herbal remedies” be found?
Consult reputable sources, such as scientific journals, peer-reviewed publications, and evidence-based databases (e.g., Natural Medicines Database). Seek guidance from qualified healthcare professionals with expertise in botanical medicine. Avoid relying solely on anecdotal evidence or unsubstantiated claims found on the internet.
In summary, responsible and informed use involves careful consideration of safety, efficacy, and quality, along with professional guidance.
The subsequent section will delve into potential challenges and future directions within the field of contemporary botanical therapeutics.
Conclusion
The preceding exploration has elucidated the multifaceted nature of plant-derived treatments within contemporary healthcare. Efficacy validation through scientific research, stringent safety protocols, standardized production methodologies, and comprehensive regulatory oversight are critical for responsible integration. The convergence of traditional knowledge and evidence-based practice defines the responsible application of these treatments. Further, informed decision-making on the part of both practitioners and patients constitutes an indispensable element.
Continued rigorous investigation and critical evaluation remain paramount. The sustained advancement of botanical science necessitates a commitment to ethical sourcing, sustainable practices, and transparent communication. These factors are integral to unlocking the full therapeutic potential while mitigating potential risks associated with “modern herbal remedies”, ultimately ensuring patient well-being and fostering confidence in their therapeutic value.


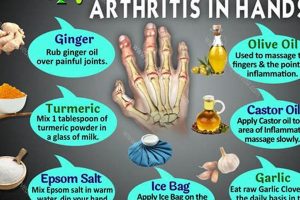
![Top: Best Herbal Remedy for Arthritis [Guide] The Ultimate Herbal Remedies Guide: Natural Healing for a Healthier Life Top: Best Herbal Remedy for Arthritis [Guide] | The Ultimate Herbal Remedies Guide: Natural Healing for a Healthier Life](https://umangherbals.com/wp-content/uploads/2026/03/th-27-300x200.jpg)