The term refers to the use of plant-derived substances to alleviate the symptoms associated with a chronic respiratory disease characterized by inflammation and narrowing of the airways. These substances are often employed as complementary or alternative therapeutic approaches, supplementing or replacing conventional pharmaceutical interventions. As an example, some individuals utilize extracts from certain plants, prepared as teas or supplements, with the intention of reducing airway constriction and inflammation during episodes of respiratory distress.
The application of botanicals in managing respiratory conditions has a long and varied history across numerous cultures. Their perceived value stems from anecdotal evidence and traditional medical systems that attribute specific medicinal properties to various plant species. Proponents suggest potential advantages, such as a reduced risk of side effects compared to some pharmaceutical options and the potential for synergistic effects from multiple compounds within a single plant. However, rigorous scientific validation of their efficacy and safety remains a crucial consideration.
The subsequent discussion will explore the prominent botanicals used, examine the available scientific evidence supporting their use, and address critical safety considerations and potential interactions with conventional medications. This will provide a balanced perspective on the role these substances may play in managing the respiratory disease.
Guidance on Botanical Approaches for Respiratory Wellness
The following recommendations offer a considered approach to exploring complementary botanical options, alongside conventional treatments, for managing respiratory conditions. It is imperative to prioritize informed decision-making and professional medical consultation.
Tip 1: Prioritize Consultation with a Qualified Healthcare Provider: Before initiating any complementary regimen, engage in thorough discussion with a physician, pulmonologist, or qualified herbalist. This ensures the chosen botanical remedies are appropriate for the individual’s specific condition, medical history, and current medications.
Tip 2: Research the Scientific Evidence: Investigate the existing scientific literature pertaining to the efficacy and safety of specific botanicals. Peer-reviewed studies published in reputable journals provide the most reliable information. Be wary of anecdotal claims or unsubstantiated marketing materials.
Tip 3: Source Products from Reputable Manufacturers: Select botanical products from established companies that adhere to stringent quality control standards, such as Good Manufacturing Practices (GMP). This minimizes the risk of contamination or adulteration and ensures accurate labeling of ingredients and dosages.
Tip 4: Begin with Low Dosages and Monitor for Adverse Reactions: When introducing a new botanical remedy, start with a conservative dosage, as suggested by a qualified practitioner or product label. Closely monitor for any potential side effects or allergic reactions. Discontinue use immediately if adverse symptoms arise.
Tip 5: Be Aware of Potential Drug Interactions: Certain botanicals can interact with conventional medications, potentially altering their effectiveness or increasing the risk of adverse effects. Inform the healthcare provider of all supplements and medications being taken to assess potential interactions.
Tip 6: Consider the Method of Administration: Botanicals can be administered in various forms, including teas, capsules, tinctures, and essential oils. The method of administration can influence the rate of absorption and bioavailability of active compounds. Choose a method that is appropriate for the individual’s condition and tolerance.
Tip 7: Emphasize a Holistic Approach to Wellness: Botanical remedies are most effective when integrated into a comprehensive approach to health, which includes a balanced diet, regular exercise, stress management techniques, and avoidance of respiratory irritants.
Adhering to these guidelines promotes responsible exploration of botanical options, prioritizing safety and informed decision-making. The integration of complementary modalities should always be conducted under the guidance of qualified healthcare professionals.
The subsequent sections of this document will delve into specific botanical substances commonly employed and provide a deeper analysis of their potential benefits and risks.
1. Efficacy Evaluation
The assessment of efficacy forms the cornerstone of determining the potential utility of botanical interventions for respiratory conditions. It necessitates a rigorous and systematic approach to ascertain whether a given substance demonstrably improves respiratory function and reduces symptom burden. This evaluation is particularly critical given the variability in plant composition and the potential for placebo effects.
- Randomized Controlled Trials (RCTs)
RCTs represent the gold standard for evaluating efficacy. These studies involve randomly assigning participants with respiratory conditions to receive either a botanical intervention or a placebo (or standard treatment). By comparing outcomes between groups, researchers can determine whether the intervention produces statistically significant and clinically meaningful improvements in lung function, symptom scores, or medication usage. For instance, an RCT assessing the effects of Andrographis paniculata might compare peak expiratory flow rates and symptom diaries between the treatment and placebo groups. The results of RCTs provide the strongest evidence for or against the efficacy of a botanical substance.
- Meta-Analyses and Systematic Reviews
Meta-analyses and systematic reviews synthesize data from multiple RCTs to provide a comprehensive overview of the evidence base. These analyses pool results from individual studies to increase statistical power and reduce the risk of bias. A systematic review might examine all available RCTs on the use of Glycyrrhiza glabra (licorice) to identify consistent trends and overall effect sizes. The conclusions drawn from these analyses offer a more robust assessment of efficacy than any single study can provide.
- Objective Outcome Measures
Reliable efficacy evaluation relies heavily on objective outcome measures that are independent of subjective bias. Examples include spirometry (measuring lung volumes and airflow), fractional exhaled nitric oxide (FeNO, indicating airway inflammation), and blood tests measuring inflammatory markers. Utilizing these measures provides a more accurate assessment of physiological changes resulting from botanical use. Relying solely on patient-reported outcomes can be problematic due to the potential for placebo effects and reporting biases.
- Long-Term Studies
Many botanical interventions are intended for long-term use as part of a comprehensive management strategy. Therefore, it is crucial to conduct studies that assess the long-term efficacy and safety of these substances. These studies should evaluate sustained improvements in respiratory function, reduction in exacerbation frequency, and absence of cumulative adverse effects over extended periods. Short-term studies may provide misleading results if the botanical has a delayed or subtle effect.
The integration of these evaluation methods is essential for generating credible insights into the potential role of botanical remedies in addressing respiratory conditions. While traditional use and anecdotal reports may suggest benefits, a systematic and scientifically rigorous approach is necessary to validate efficacy claims and ensure that patients receive treatments that are both safe and effective. The findings of efficacy evaluations ultimately inform clinical practice and guide the development of evidence-based recommendations for botanical use.
2. Safety Profile
The safety profile constitutes a critical dimension in the evaluation of botanical interventions for respiratory conditions. Assessing potential adverse effects, drug interactions, and contraindications is paramount to ensuring that these remedies are used responsibly and do not compromise patient well-being. Given the complex chemical compositions of many plants and the potential for variable responses among individuals, a thorough understanding of safety considerations is essential.
- Potential Adverse Effects
Botanical substances, like any therapeutic agent, can elicit a range of adverse effects. These may include allergic reactions (skin rashes, urticaria, anaphylaxis), gastrointestinal disturbances (nausea, vomiting, diarrhea), and neurological symptoms (headache, dizziness). The severity of these effects can vary depending on the individual’s sensitivity, the dosage used, and the specific botanical. For example, excessive consumption of licorice root may lead to sodium and water retention, potentially exacerbating hypertension or heart failure. Awareness of these potential adverse effects is crucial for prompt recognition and management.
- Drug Interactions
Botanical substances can interact with conventional medications, either enhancing or diminishing their effects. These interactions may occur through various mechanisms, including alterations in drug metabolism, absorption, or excretion. For instance, St. John’s Wort, an herb used for mood disorders, can induce hepatic enzymes that accelerate the metabolism of certain medications, potentially reducing their effectiveness. Individuals taking conventional medications for respiratory conditions, such as corticosteroids or bronchodilators, should exercise particular caution and consult with their healthcare provider to assess potential interactions.
- Contraindications
Specific medical conditions or physiological states may contraindicate the use of certain botanical substances. For example, pregnant or breastfeeding women should generally avoid using botanical remedies due to the potential for teratogenic or adverse effects on the infant. Individuals with liver or kidney disease may be more susceptible to the toxic effects of certain plants. Moreover, certain botanicals may be contraindicated in individuals with bleeding disorders or those undergoing surgery. Careful evaluation of the patient’s medical history is essential to identify potential contraindications.
- Quality Control and Contamination
The safety profile of botanical remedies is also influenced by product quality and potential contamination. Poor manufacturing practices can lead to products contaminated with heavy metals, pesticides, or other adulterants. Misidentification of plant species can result in the use of incorrect or even toxic botanicals. Therefore, it is crucial to source botanical products from reputable manufacturers that adhere to stringent quality control standards and provide accurate labeling of ingredients and dosages.
The multifaceted nature of the safety profile underscores the importance of a cautious and informed approach to the use of botanical interventions for respiratory conditions. While these remedies may offer potential benefits, a thorough assessment of risks and potential interactions is essential to ensure patient safety and optimize therapeutic outcomes. Consultation with qualified healthcare providers, including physicians, pharmacists, and herbalists, is strongly recommended.
3. Dosage Standardization
Dosage standardization is a critical factor in the safe and effective utilization of botanicals for respiratory conditions. Variability in plant composition and extraction methods necessitates precise dosage control to minimize adverse effects and maximize therapeutic benefits. In the context of “asthma herbal remedies,” inconsistent dosing can lead to either treatment failure or potential toxicity.
- Active Compound Identification and Quantification
Dosage standardization begins with the identification and quantification of the active chemical constituents responsible for the therapeutic effects. Without knowing the concentration of these compounds in a botanical preparation, it is impossible to achieve consistent dosing. For example, Glycyrrhiza glabra (licorice) contains glycyrrhizin, which exerts anti-inflammatory effects. Standardized extracts should specify the percentage of glycyrrhizin present. This specification is essential for replication of research findings and ensuring predictable clinical outcomes.
- Extraction Method Influence
The method used to extract active compounds from plant material can significantly influence the final concentration of these compounds in the product. Aqueous extracts (teas), alcoholic tinctures, and supercritical fluid extracts can yield different ratios of active and inactive constituents. Dosage recommendations must account for the extraction method used. An aqueous extract, for instance, may require a larger volume to achieve the same therapeutic effect as a concentrated tincture.
- Bioavailability Considerations
Dosage standardization must also consider the bioavailability of active compounds, which refers to the extent to which they are absorbed and utilized by the body. Some compounds may have limited bioavailability when taken orally, requiring higher doses to achieve therapeutic concentrations in the target tissues. For example, curcumin, a compound found in turmeric ( Curcuma longa), has poor bioavailability. Formulations that enhance curcumin absorption, such as those containing piperine (from black pepper), may allow for lower effective dosages.
- Patient-Specific Factors
Dosage requirements can vary depending on individual factors such as age, weight, liver and kidney function, and the severity of the respiratory condition. Pediatric patients or individuals with impaired organ function may require lower dosages to avoid toxicity. Furthermore, interactions with conventional medications can affect the metabolism and excretion of botanical compounds, necessitating dosage adjustments. A healthcare provider should always consider these factors when recommending dosages of “asthma herbal remedies”.
Precise dosage standardization of botanicals used as “asthma herbal remedies” is paramount for ensuring both safety and effectiveness. A lack of standardization introduces significant variability in treatment outcomes and increases the risk of adverse events. Therefore, reliance on products with clearly defined active constituents and evidence-based dosage guidelines is crucial for responsible use.
4. Interaction Potential
The interaction potential inherent in “asthma herbal remedies” is a critical determinant of their safety and efficacy when used in conjunction with conventional asthma treatments. Botanical substances possess diverse chemical constituents that can either augment or diminish the effects of pharmaceuticals, leading to unintended consequences. Cause-and-effect relationships within this context are often complex, arising from alterations in drug metabolism, absorption, distribution, or excretion. Understanding these interaction pathways is paramount to preventing adverse events and optimizing therapeutic outcomes. An instance of significant interaction potential is the use of certain herbal products containing constituents that induce cytochrome P450 enzymes in the liver. These enzymes are responsible for metabolizing many asthma medications, such as corticosteroids and beta-agonists. Induction of these enzymes can lead to a decrease in the concentration of these drugs in the body, potentially resulting in a loss of asthma control. Conversely, some herbal constituents may inhibit these enzymes, leading to elevated drug levels and an increased risk of side effects.
Further complicating the assessment of interaction potential is the inherent variability in the composition of herbal products. Factors such as plant species, growing conditions, harvesting methods, and extraction processes can influence the concentration of active constituents. This variability makes it challenging to predict the magnitude and clinical significance of interactions. Moreover, many individuals use multiple herbal products simultaneously, increasing the likelihood of complex interactions. From a practical standpoint, healthcare providers must routinely inquire about the use of herbal remedies during patient consultations. Thorough medication reconciliation, including both prescription and over-the-counter drugs as well as supplements, is essential. When potential interactions are identified, adjustments to medication dosages or alternative therapeutic strategies may be necessary. Resources such as drug interaction databases and consultations with pharmacists or herbalists can provide valuable guidance in assessing and managing these risks.
In summary, the interaction potential represents a non-negligible facet of “asthma herbal remedies” that directly influences patient safety and treatment efficacy. The inherent complexity of these interactions underscores the necessity of a cautious and informed approach. Challenges persist in predicting and managing these interactions due to the variability of herbal products and the potential for multiple concurrent exposures. By prioritizing comprehensive medication reconciliation, utilizing available resources, and maintaining open communication between patients and healthcare providers, the risks associated with the interaction potential of “asthma herbal remedies” can be effectively mitigated. This understanding also links to the broader theme of integrating complementary and alternative therapies responsibly within a conventional medical framework.
5. Quality Control
Quality control in the realm of “asthma herbal remedies” is not merely a procedural formality but a critical safeguard for patient health and therapeutic efficacy. Due to the inherent complexities in plant-based medicine, rigorous quality control measures are indispensable to ensure consistency, purity, and safety. The absence of such controls can render botanical treatments ineffective or, worse, harmful.
- Botanical Authentication
Correct species identification is fundamental. Misidentification can lead to the use of incorrect, potentially toxic, plants. Reputable manufacturers employ trained botanists and utilize techniques such as macroscopic and microscopic examination, as well as DNA barcoding, to verify the identity of plant material. An example of the importance of this lies in ensuring that Echinacea purpurea is not substituted with another, less potent or even harmful, Echinacea species. For “asthma herbal remedies,” this eliminates the risk of using a plant that lacks the intended therapeutic effect or contains harmful compounds.
- Contaminant Testing
Herbal products are susceptible to contamination from various sources, including heavy metals (lead, mercury, arsenic), pesticides, herbicides, and microbial pathogens (bacteria, fungi). Stringent testing protocols, employing techniques such as atomic absorption spectroscopy and chromatography, are necessary to ensure that products meet established safety standards for these contaminants. For instance, rigorous testing would detect unacceptable levels of lead in a batch of Ginkgo biloba, preventing its distribution as an “asthma herbal remedy” and protecting consumers from heavy metal toxicity.
- Active Constituent Standardization
To ensure consistent therapeutic effects, the concentration of key active constituents must be standardized across different batches of a herbal product. This involves quantitative analysis using techniques such as high-performance liquid chromatography (HPLC) to determine the amount of specific compounds, like glycyrrhizin in licorice ( Glycyrrhiza glabra). Standardization ensures that each dose delivers a predictable amount of the active constituent, reducing variability in treatment response, a critical factor in managing respiratory symptoms with “asthma herbal remedies.”
- Stability Testing
The potency and purity of herbal products can degrade over time due to factors such as temperature, light, and humidity. Stability testing involves storing products under controlled conditions and periodically analyzing them to assess changes in active constituent levels and the formation of degradation products. This ensures that the product remains effective and safe throughout its shelf life. Proper stability testing of an “asthma herbal remedy” ensures that it will maintain its advertised potency until its expiration date, allowing consumers to rely on the labeled dosage for symptom management.
These facets of quality control, when rigorously implemented, provide a framework for ensuring the safety and efficacy of “asthma herbal remedies.” By focusing on botanical authentication, contaminant testing, active constituent standardization, and stability testing, manufacturers can offer products that meet established quality standards and provide consumers with a reasonable expectation of therapeutic benefit. However, even with robust quality control, individual responses to botanical treatments can vary, emphasizing the need for informed decision-making and consultation with healthcare professionals.
6. Individual Variability
Individual variability plays a substantial role in determining the response to “asthma herbal remedies.” Factors inherent to each person’s physiology and medical history influence the efficacy and safety of these treatments, making a uniform outcome unlikely. This variability necessitates personalized approaches rather than blanket recommendations.
- Genetic Predisposition
Genetic factors influence an individual’s metabolism, immune response, and susceptibility to respiratory conditions. Genetic polymorphisms in enzymes responsible for metabolizing botanical compounds can affect drug clearance rates, leading to altered drug concentrations and therapeutic effects. For example, variations in cytochrome P450 enzymes may impact the metabolism of constituents in certain herbal remedies, resulting in either enhanced or diminished efficacy. This can translate to varied responses in different individuals using the same “asthma herbal remedy,” with some experiencing symptom relief while others do not.
- Physiological State
Age, body weight, organ function, and concurrent medical conditions influence how the body processes and responds to botanical substances. Pediatric and geriatric populations may exhibit altered drug metabolism and increased sensitivity to side effects. Individuals with liver or kidney impairment may experience reduced clearance of herbal compounds, leading to increased toxicity risks. A heavier individual may require a higher dose of an “asthma herbal remedy” compared to a lighter person to achieve the same therapeutic effect, assuming similar metabolic rates. Consideration of these physiological states is crucial for safe and effective application.
- Environmental Factors
Exposure to environmental pollutants, allergens, and dietary habits can impact the severity of respiratory symptoms and the response to treatment. Individuals living in areas with high levels of air pollution may exhibit increased airway inflammation and reduced responsiveness to anti-inflammatory botanical compounds. Similarly, dietary factors, such as the consumption of processed foods or a deficiency in essential nutrients, can influence immune function and exacerbate respiratory symptoms, thereby affecting the perceived effectiveness of “asthma herbal remedies.”
- Gut Microbiome Composition
The composition of the gut microbiome can influence the metabolism and bioavailability of botanical compounds. Certain gut bacteria can metabolize herbal constituents, altering their pharmacological activity. For example, some bacteria can convert inactive compounds into active metabolites, while others can degrade active compounds, reducing their effectiveness. Variability in gut microbiome composition among individuals can therefore lead to differences in the systemic exposure and therapeutic effects of “asthma herbal remedies.” This underscores the importance of considering gut health when assessing individual responses to botanical treatments.
The interconnectedness of these factors highlights the intricate relationship between individual characteristics and the response to “asthma herbal remedies.” While certain botanical compounds may exhibit promising therapeutic potential, the actual outcome hinges on a complex interplay of genetics, physiology, environmental influences, and the gut microbiome. A personalized approach, informed by a comprehensive assessment of these variables, is essential to optimize the safety and efficacy of botanical interventions for respiratory conditions.
Frequently Asked Questions
This section addresses common inquiries regarding botanical approaches to respiratory management. The information provided is intended for educational purposes and should not be interpreted as medical advice. Consultation with a qualified healthcare professional is strongly recommended before initiating any new treatment regimen.
Question 1: Are “asthma herbal remedies” a substitute for conventional medical treatment?
No, botanical interventions should not be considered a replacement for conventional medical care. Asthma is a chronic condition requiring ongoing management by a qualified healthcare provider. Herbal remedies may be used as complementary therapies, but should not supersede prescribed medications or treatment plans.
Question 2: Is the term “natural” synonymous with “safe” when referring to herbal remedies?
The designation “natural” does not guarantee safety. Botanical substances can possess potent pharmacological activity and may cause adverse effects or interact with conventional medications. A thorough assessment of potential risks and benefits is necessary, regardless of a product’s purported “natural” origin.
Question 3: How can the quality and purity of herbal products be assured?
Select products from reputable manufacturers that adhere to Good Manufacturing Practices (GMP) and provide certificates of analysis (COAs) verifying the identity and purity of ingredients. Third-party testing and independent verification can provide additional assurance of product quality.
Question 4: What are the potential risks associated with using “asthma herbal remedies”?
Potential risks include allergic reactions, drug interactions, and adverse effects stemming from contamination or misidentification of plant species. Pregnant or breastfeeding women, individuals with underlying medical conditions, and those taking prescription medications should exercise particular caution.
Question 5: How can the effectiveness of “asthma herbal remedies” be determined?
Assess effectiveness through objective measures, such as pulmonary function tests and symptom diaries, in conjunction with a healthcare provider. Subjective improvements should be corroborated by objective findings to ensure that the treatment is providing tangible benefits.
Question 6: Are there legal regulations governing the production and sale of “asthma herbal remedies”?
The regulatory landscape for herbal products varies by jurisdiction. In many regions, herbal remedies are classified as dietary supplements, which are subject to less stringent regulations than pharmaceutical drugs. Consumers should be aware of these regulatory differences and exercise caution when selecting herbal products.
This information serves as a starting point for understanding the complexities of botanical approaches to respiratory management. Further research and consultation with healthcare professionals are essential for informed decision-making.
The following section will explore case studies that illustrate the real-world application of these principles.
Conclusion
The preceding analysis underscores the multifaceted nature of “asthma herbal remedies.” Exploration of this topic reveals a landscape characterized by potential benefits tempered by significant risks and uncertainties. The importance of evidence-based decision-making, rigorous quality control, and individualized assessment cannot be overstated. The information detailed aims to provide a comprehensive, balanced perspective, moving beyond simplistic endorsements or dismissals of botanical interventions for respiratory conditions.
The responsible integration of herbal therapies into asthma management necessitates continued scientific investigation, enhanced regulatory oversight, and a commitment to open communication between patients and healthcare providers. Further research focusing on well-defined botanical products and rigorous clinical trials is essential to clarify the role of these substances in the therapeutic landscape. Ultimately, a cautious, informed approach that prioritizes patient safety and collaborative care will best serve those seeking complementary options for asthma management.


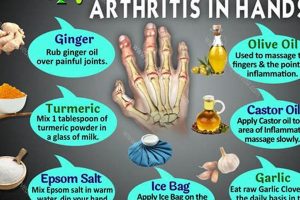
![Top: Best Herbal Remedy for Arthritis [Guide] The Ultimate Herbal Remedies Guide: Natural Healing for a Healthier Life Top: Best Herbal Remedy for Arthritis [Guide] | The Ultimate Herbal Remedies Guide: Natural Healing for a Healthier Life](https://umangherbals.com/wp-content/uploads/2026/03/th-27-300x200.jpg)