A compilation of plant-derived therapeutic agents, categorized often by usage, origin, or chemical constituents, provides a structured inventory for practitioners and researchers. These inventories can range from specific regional applications to global compendiums encompassing a broad range of plant species. An example might be an organized registry of plants traditionally employed in Traditional Chinese Medicine, detailing their properties and applications.
Such organized collections offer several benefits, including facilitating informed decision-making in healthcare, promoting the preservation of traditional knowledge, and providing a valuable resource for pharmacological research. Historically, these catalogs served as vital repositories of empirical knowledge passed down through generations, forming the basis for many modern pharmaceuticals.
The subsequent sections will delve into the criteria for inclusion in such compendiums, address the challenges associated with standardization and quality control, and examine the role of these inventories in contemporary healthcare practices.
Guidance on Compiling Information Regarding Plant-Based Remedies
The following guidelines are intended to assist in the creation and utilization of resources pertaining to therapeutic plants. Accuracy, validation, and adherence to regulatory standards are of paramount importance.
Tip 1: Prioritize Scientific Validation: Include only those preparations with documented efficacy and safety profiles, based on peer-reviewed research and established pharmacological principles. Avoid anecdotal evidence and unsubstantiated claims.
Tip 2: Emphasize Accurate Botanical Identification: Employ standardized nomenclature and provide verifiable sources for plant identification. Include details such as Latin binomial, common names, and voucher specimen information where available.
Tip 3: Document Traditional Use with Context: If including information on traditional applications, provide the cultural and historical context. Differentiate between traditional knowledge and established medical practices.
Tip 4: Clearly Define Preparation Methods: Specify the extraction techniques, solvents used, and standardization protocols employed in the preparation of each remedy. This ensures reproducibility and consistency in formulation.
Tip 5: Outline Potential Adverse Effects and Interactions: Provide comprehensive details regarding potential side effects, contraindications, and interactions with other medications, both herbal and pharmaceutical.
Tip 6: Adhere to Regulatory Compliance: Ensure compliance with relevant regulations and guidelines governing the manufacture, distribution, and sale of plant-derived therapeutic agents in the applicable jurisdiction.
Tip 7: Regularly Update Information: Plant-based medicine is an evolving field. Regularly review and update the resource to reflect new research findings, regulatory changes, and emerging safety concerns.
Tip 8: Provide Dosage and Administration Guidelines: Offer clear and concise dosage recommendations based on available evidence. Consider factors such as age, weight, and underlying health conditions.
By adhering to these principles, a more reliable and valuable resource is created, promoting responsible use and informed decision-making within the realm of plant-based therapeutics. The following sections will address the challenges inherent in maintaining accurate and up-to-date information.
1. Botanical Identification
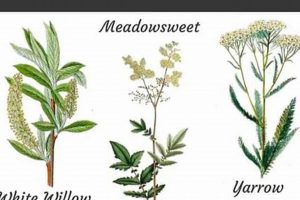
Botanical identification forms the foundational element for any authoritative compendium of plant-derived therapeutic agents. Accurate species determination is not merely a matter of scientific rigor; it directly impacts patient safety and treatment efficacy. Misidentification can lead to the use of the wrong plant, potentially resulting in adverse effects, lack of therapeutic benefit, or even toxicity. The inclusion of a plant within a list of herbal medicines necessitates unequivocal verification of its identity using standardized nomenclature, such as Latin binomials, and verifiable voucher specimens. For example, Digitalis purpurea (foxglove) is a well-known source of cardiac glycosides; however, confusing it with other plants could lead to ineffective treatment or dangerous consequences if a substitute lacks the same active compounds or contains different, potentially harmful, substances.
The challenges associated with botanical identification within the context of herbal medicine compilations are multifaceted. Variations in plant morphology due to environmental factors, geographical location, and growth stage can complicate the identification process. Furthermore, the use of common names, which vary regionally and often lack precision, further compounds the issue. To mitigate these challenges, stringent quality control measures, including macroscopic and microscopic examination, chemical fingerprinting, and DNA barcoding, are increasingly employed to ensure accurate and reliable botanical identification. These techniques help to differentiate between closely related species and detect adulteration, thereby safeguarding the integrity of the information presented.
In conclusion, precise botanical identification is indispensable for compiling a credible and reliable catalog of plant-based therapies. It serves as the cornerstone upon which all other information regarding a plant’s therapeutic potential and safety profile is built. By prioritizing accurate identification and employing robust quality control measures, these resources can effectively serve healthcare professionals, researchers, and the public, promoting the responsible and safe use of plant-derived medicines. The challenge lies in consistently applying these rigorous standards across all entries, particularly for plants sourced from diverse geographical regions and those with complex taxonomic relationships.
2. Chemical Constituents
The presence and concentration of specific chemical constituents define the therapeutic potential and pharmacological activity of any plant included in a compendium of herbal remedies. An understanding of these constituents is crucial for standardization, dosage determination, and the prediction of potential interactions or adverse effects.
- Active Compounds and Their Role
Plants contain a diverse array of chemical compounds, including alkaloids, flavonoids, terpenes, and glycosides, each with unique pharmacological properties. For example, the alkaloid quinine, derived from the Cinchona tree, exhibits antimalarial activity. The inclusion of information on the identified active compounds allows practitioners to understand the mechanism of action and potential therapeutic applications of each plant.
- Standardization and Quality Control
The concentration of active constituents can vary significantly depending on factors such as plant genetics, growing conditions, and harvesting methods. Standardized extracts, which guarantee a consistent level of one or more active compounds, are essential for ensuring reproducible therapeutic effects. A well-maintained compilation will specify the standardization parameters used for each plant-based medicine.
- Synergistic Effects and Interactions
The therapeutic effects of plant-based medicines are often attributed to the synergistic interactions of multiple chemical constituents, rather than a single isolated compound. It is crucial to consider the interactions between different plant constituents and with other medications. A detailed analysis of these interactions is vital for patient safety.
- Potential for Toxicity
Some chemical constituents present in plants can exhibit toxic effects, even at low concentrations. A responsible directory of herbal remedies must include comprehensive information on potential toxicities, contraindications, and safe dosage ranges. For instance, pyrrolizidine alkaloids, found in certain plant species, can cause liver damage.
In summary, detailed knowledge of the chemical constituents of each plant is paramount to the compilation of a reliable and informative repository of plant-based therapies. This knowledge informs proper usage, facilitates standardization, and helps to mitigate potential risks associated with plant-derived medicines. Omission of such detailed information renders the resource incomplete and potentially hazardous.
3. Traditional Uses
The integration of traditional uses into a “list of herbal medicines” provides essential context for understanding the potential therapeutic applications of plant-based remedies. Traditional use represents a historical record of empirical observation and accumulated knowledge regarding the effects of plants on human health. Its inclusion is not merely for historical interest, but as a potential indicator of pharmacological activity and safety. For instance, the use of Artemisia annua in traditional Chinese medicine as a treatment for fever provided the foundation for the discovery of artemisinin, a highly effective antimalarial drug. This demonstrates the direct impact of traditional knowledge on modern medicine.
However, the inclusion of traditional uses necessitates careful evaluation and critical analysis. Not all traditional applications have been scientifically validated, and some may be based on cultural beliefs or anecdotal evidence rather than empirical evidence. Therefore, a robust inventory of plant-based medicines should clearly distinguish between traditional uses supported by scientific research and those lacking such validation. Additionally, it is imperative to document the specific preparation methods, dosages, and routes of administration associated with traditional applications, as these factors can significantly influence the efficacy and safety of the remedy. The traditional use of Hypericum perforatum (St. John’s Wort) as a wound healer, for example, differs significantly from its modern application as an antidepressant, highlighting the importance of contextual specificity.
In conclusion, the inclusion of traditional uses enriches a “list of herbal medicines,” offering insights into potential therapeutic applications and guiding future research. However, it is crucial to approach this information with a critical eye, ensuring that traditional knowledge is evaluated in conjunction with scientific evidence and documented with sufficient detail to inform safe and effective use. The challenge lies in bridging the gap between traditional wisdom and modern scientific understanding, translating empirical observations into evidence-based practices.
4. Pharmacological Actions
The pharmacological actions of a plant, detailing its effects on biological systems, are fundamental to a comprehensive “list of herbal medicines”. The presence or absence of verified pharmacological activity is a primary determinant of whether a plant qualifies for inclusion. Documentation of these actions, elucidated through in vitro, in vivo, and clinical studies, provides the scientific rationale for its purported therapeutic benefits. For example, the listing of Salix alba (white willow bark) is justified by its demonstrated anti-inflammatory and analgesic properties, stemming from the presence of salicin, a precursor to salicylic acid. This cause-and-effect relationship between chemical constituents and observed physiological effects is a prerequisite for informed medicinal use.
Understanding pharmacological actions enables healthcare professionals to make evidence-based decisions regarding the appropriate use of herbal remedies. It allows for the prediction of potential drug interactions, contraindications, and adverse effects. Without this information, the use of herbal medicines becomes largely empirical and potentially dangerous. For instance, knowing that Hypericum perforatum (St. John’s Wort) is a CYP450 inducer allows clinicians to anticipate and manage potential interactions with prescription medications, such as warfarin or oral contraceptives. This knowledge translates directly into improved patient safety and therapeutic outcomes.
In summary, a credible “list of herbal medicines” must prioritize the inclusion of well-defined pharmacological actions supported by scientific evidence. This information provides the basis for rational therapeutic application, facilitates the identification of potential risks, and enables the integration of herbal remedies into conventional medical practice. The ongoing challenge lies in rigorously evaluating the pharmacological properties of traditionally used plants, ensuring that claims of efficacy are substantiated by robust scientific data and that potential safety concerns are thoroughly investigated.
5. Safety Profiles
The establishment of comprehensive safety profiles is paramount for any credible compendium of plant-based therapeutic agents. Inclusion within a “list of herbal medicines” necessitates a rigorous assessment of potential risks, contraindications, and adverse effects associated with each plant species. This assessment informs appropriate usage and mitigates potential harm to patients.
- Adverse Reactions and Toxicity
Documenting potential adverse reactions, ranging from mild allergic responses to severe toxicities, is crucial. The listing must provide details on the specific symptoms, mechanisms of action, and effective treatments for adverse effects. For example, certain plants containing pyrrolizidine alkaloids are known to cause liver damage with prolonged use. The inclusion of this information, along with dosage limits and contraindications, is essential for responsible use.
- Drug Interactions
Many plant-based remedies can interact with prescription medications, potentially altering their efficacy or increasing the risk of adverse effects. A robust safety profile will identify known and potential drug interactions, specifying the mechanisms involved (e.g., enzyme induction or inhibition, altered absorption). For instance, St. John’s Wort (Hypericum perforatum) is a known CYP450 inducer, which can reduce the effectiveness of numerous medications, including oral contraceptives and anticoagulants.
- Contraindications and Precautions
Specific contraindications, such as pregnancy, lactation, or pre-existing medical conditions, must be clearly delineated within the safety profile. Certain plants may be unsafe for specific populations due to potential teratogenic effects, hormonal activity, or interference with existing medical treatments. For example, Saw Palmetto (Serenoa repens), commonly used for prostate health, is contraindicated in individuals taking anticoagulant medications due to its potential to increase bleeding risk.
- Dosage and Duration of Use
The safety profile must include clear guidelines on appropriate dosage and duration of use. Exceeding recommended dosages or using a plant for prolonged periods can increase the risk of adverse effects. Information on potential cumulative toxicity and safe dosage ranges for different populations (e.g., children, elderly) is also crucial. For instance, excessive consumption of licorice root (Glycyrrhiza glabra) can lead to hypertension and electrolyte imbalances.
In summary, thorough documentation of safety profiles is indispensable for any reliable compendium of plant-based medicines. This information empowers healthcare professionals and patients to make informed decisions regarding the safe and effective use of herbal remedies. Neglecting the safety aspects compromises the integrity of the list and poses a significant risk to public health. The continual updating of these profiles with new research findings and adverse event reports is crucial for maintaining their accuracy and relevance.
6. Regulatory Status
The regulatory status of plant-based remedies exerts a substantial influence on the composition and utility of any “list of herbal medicines.” Governmental regulations governing the manufacture, distribution, and marketing of these products dictate which substances can be legally included and under what conditions they can be utilized. These regulations vary significantly across jurisdictions, impacting the availability, quality, and safety of herbal products. For example, a plant authorized for use as a dietary supplement in one country may be classified as a prescription drug or prohibited substance in another. This variance directly affects which plants can be included in a given list designed for a specific geographical region.
The cause-and-effect relationship between regulatory oversight and the content of such inventories is evident. Stricter regulatory frameworks, demanding evidence of safety and efficacy through clinical trials, result in a more limited selection of plants included in approved directories. Conversely, less stringent regulations may allow for the inclusion of a wider range of plants, but with a higher risk of safety concerns or unsubstantiated claims. Consider the case of Aristolochia species. These plants are widely restricted due to their association with nephrotoxicity and cancer, leading to their exclusion from most regulated “list of herbal medicines,” while they may still appear in inventories compiled in regions with less stringent controls. The practical significance of understanding these nuances is crucial for healthcare providers and consumers, as it informs their choices and guides them towards safe and effective plant-based therapies.
In summary, regulatory status serves as a critical filter in the compilation of any “list of herbal medicines,” shaping its content based on legal and safety considerations. Navigating the complex landscape of international regulations is essential for creating accurate and reliable repositories of plant-derived remedies. Ongoing harmonization efforts aim to standardize regulations and promote the responsible use of herbal medicines globally, ultimately improving the quality and safety of available resources. However, until such harmonization is achieved, a thorough understanding of regional regulations remains vital for the proper utilization of these inventories.
7. Quality Control
Quality control constitutes a critical aspect in the creation and maintenance of any reliable compendium of plant-based therapeutic agents. Its implementation ensures the accuracy, consistency, and safety of products included on a “list of herbal medicines,” safeguarding public health and promoting responsible usage. Absence of rigorous quality control measures can undermine the credibility of such inventories, leading to misidentification, adulteration, and potential harm.
- Botanical Authentication
Accurate identification of plant species forms the bedrock of quality control. Misidentification can result in the inclusion of incorrect or even toxic plants on a “list of herbal medicines.” Techniques such as macroscopic and microscopic examination, DNA barcoding, and chemical fingerprinting are employed to verify the identity of plant materials and to detect adulteration. For example, substitution of Echinacea purpurea with other, less potent Echinacea species would compromise the therapeutic efficacy of products derived from the plant.
- Contaminant Testing
Plant-based remedies are susceptible to contamination from various sources, including heavy metals, pesticides, microbial pathogens, and mycotoxins. Quality control procedures mandate the testing of raw materials and finished products to ensure that contaminant levels remain within acceptable limits. The presence of heavy metals, such as lead or mercury, in products listed on a “list of herbal medicines” would pose a significant health risk to consumers.
- Active Constituent Standardization
The concentration of active constituents can vary significantly depending on factors such as growing conditions, harvesting techniques, and storage methods. Standardization ensures consistent levels of key compounds, leading to reproducible therapeutic effects. Quality control protocols require the quantification of active constituents using analytical techniques such as high-performance liquid chromatography (HPLC). For example, standardized extracts of Hypericum perforatum (St. John’s Wort) guarantee a specific concentration of hypericin, a key antidepressant compound.
- Good Manufacturing Practices (GMP)
Adherence to Good Manufacturing Practices (GMP) is essential for ensuring the quality and safety of herbal products. GMP guidelines cover all aspects of production, from raw material sourcing to packaging and labeling. GMP compliance helps to prevent contamination, ensure proper documentation, and promote consistency in product quality. Inclusion of products sourced from GMP-certified manufacturers on a “list of herbal medicines” provides assurance to consumers regarding the quality and safety of those products.
In conclusion, quality control forms an indispensable component in the development and maintenance of reliable inventories of plant-based therapeutic agents. By implementing rigorous quality control measures at every stage of the production process, the integrity and safety of products listed on a “list of herbal medicines” can be assured. These measures not only safeguard public health but also enhance the credibility and trustworthiness of herbal medicine as a complementary and alternative therapeutic modality.
Frequently Asked Questions Regarding Compilations of Plant-Based Remedies
This section addresses common inquiries and misconceptions concerning inventories of plant-derived therapeutic agents. It aims to provide clarity and guidance for healthcare professionals and the public.
Question 1: What criteria determine inclusion in a “list of herbal medicines”?
Inclusion criteria typically encompass botanical authentication, chemical characterization, documentation of traditional use, scientific validation of pharmacological activity, a comprehensive safety profile, and adherence to relevant regulatory standards.
Question 2: How is the accuracy of botanical identification ensured in a “list of herbal medicines”?
Accuracy is maintained through the use of standardized nomenclature (Latin binomials), verifiable voucher specimens, macroscopic and microscopic examination, chemical fingerprinting, and, in some cases, DNA barcoding techniques.
Question 3: What information is typically included in the safety profile of an entry in a “list of herbal medicines”?
The safety profile typically details potential adverse reactions, drug interactions, contraindications, precautions, and dosage guidelines, along with any available information on toxicity.
Question 4: How are traditional uses distinguished from scientifically validated uses in a “list of herbal medicines”?
A clear distinction is made between traditional uses supported by peer-reviewed scientific research and those based solely on historical or anecdotal evidence. The level of scientific support is often indicated for each listed application.
Question 5: How frequently are “list of herbal medicines” updated to reflect new research or regulatory changes?
The frequency of updates varies depending on the specific inventory and its intended audience. However, reputable inventories are regularly reviewed and revised to incorporate new research findings, regulatory changes, and emerging safety concerns.
Question 6: Who is responsible for ensuring the quality and accuracy of information presented in a “list of herbal medicines”?
Responsibility typically lies with the compilers and publishers of the inventory, who may be academic institutions, regulatory agencies, or professional organizations. These entities are accountable for implementing rigorous quality control measures and ensuring the accuracy of the information presented.
The proper use and interpretation of these catalogs requires due diligence and consultation with qualified healthcare professionals. The information presented should not be considered a substitute for professional medical advice.
The subsequent section will explore the practical applications of these compilations in clinical settings.
Conclusion
The preceding sections have explored the multifaceted nature of compilations of plant-derived therapeutic agents. From botanical identification and chemical constituents to regulatory status and quality control, the factors influencing the content and reliability of such inventories have been examined. The responsible creation and utilization of “list of herbal medicines” hinges on the integration of scientific evidence, traditional knowledge, and rigorous quality assurance practices.
The continued development and refinement of these inventories are crucial for advancing the responsible integration of plant-based therapies into modern healthcare. It is imperative that healthcare professionals, researchers, and policymakers collaborate to ensure the accuracy, safety, and efficacy of information pertaining to plant-derived medicines. Diligence in upholding these standards will ultimately benefit patient outcomes and public health.