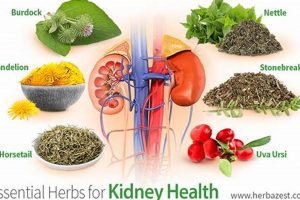
Products derived from plants, intended to provide health benefits, are often consumed to augment dietary intake. These preparations may include extracts, concentrates, or isolated compounds and are available in various forms such as capsules, tablets, powders, and liquids. A common example includes the use of Echinacea to potentially support immune function during cold and flu season.
The appeal of plant-derived wellness aids stems from a long history of traditional use and a perception of natural origin. Throughout various cultures, botanical remedies have played a significant role in healthcare practices. Their perceived benefits may include supporting overall wellness, addressing specific health concerns, and promoting preventative care. It is important to note, however, that scientific validation of these benefits varies considerably.
Understanding the composition, potential effects, and safety considerations associated with these plant-based products is crucial. The following sections will delve into different aspects of this topic, including regulation, potential interactions, and evidence-based usage, to provide a comprehensive overview.
Guidance on Plant-Derived Wellness Aids
The responsible use of plant-based wellness products requires careful consideration. The following tips offer guidance for individuals seeking to incorporate these into their health regimen.
Tip 1: Research Thoroughly: Prior to use, conduct in-depth research on the specific product. Reputable sources, such as peer-reviewed scientific literature and government health agencies, offer valuable information regarding efficacy and potential risks. For instance, understanding the active compounds and their documented effects is crucial.
Tip 2: Consult a Healthcare Professional: Engaging with a qualified healthcare provider is essential, especially for individuals with pre-existing medical conditions or those taking prescription medications. A healthcare professional can assess potential interactions and provide personalized recommendations. For example, St. John’s Wort may interact negatively with certain antidepressants.
Tip 3: Verify Product Quality: Purchase from reputable manufacturers that adhere to quality control standards. Look for third-party certifications, such as those from NSF International or USP, which indicate that the product has been independently tested for purity and potency. Avoid products with vague ingredient lists or unsubstantiated claims.
Tip 4: Understand Dosage and Administration: Adhere strictly to the recommended dosage and administration guidelines provided by the manufacturer or a healthcare professional. Overconsumption may lead to adverse effects. Furthermore, be mindful of the appropriate method of administration, as some products are designed for oral consumption while others are for topical application.
Tip 5: Monitor for Adverse Reactions: Closely monitor for any adverse reactions after initiating use. Common side effects may include gastrointestinal upset, skin irritation, or allergic reactions. Discontinue use immediately and seek medical attention if severe symptoms develop.
Tip 6: Be Aware of Potential Interactions: Be mindful of potential interactions with medications, other plant-derived products, or even certain foods. Certain products may potentiate or inhibit the effects of medications, leading to unintended consequences. For example, grapefruit juice can affect the metabolism of some drugs.
Tip 7: Consider the Source: Understanding the origin and cultivation practices of the plants used in the product can contribute to making informed choices. Opt for products sourced from sustainable and ethical suppliers.
By following these guidelines, individuals can make more informed decisions regarding the use of these products, mitigating potential risks and maximizing the possibility of positive outcomes.
The subsequent sections will explore specific categories of these products, offering further insights into their individual properties and potential benefits.
1. Botanical Origins
The botanical origin of a plant-derived wellness aid refers to the specific plant species from which it is derived. This is a foundational element, directly influencing the product’s chemical composition, potential therapeutic effects, and safety profile. Accurate identification and sourcing of the correct plant species are paramount, as different species within the same genus can exhibit vastly different properties. For example, Panax ginseng (Asian ginseng) and Panax quinquefolius (American ginseng) possess distinct chemical constituents and varying effects on the body. Misidentification or adulteration of botanical sources can render a product ineffective or even harmful.
The cultivation practices and geographic location of the source plant also significantly impact its quality and efficacy. Soil composition, climate, and harvesting methods can influence the concentration of active compounds within the plant material. Organic cultivation practices minimize exposure to pesticides and herbicides, contributing to a safer final product. Furthermore, sustainable harvesting practices are essential to ensure the long-term availability of plant resources and minimize environmental impact. The sourcing location should be transparent and verifiable to ensure authenticity and quality.
In conclusion, the botanical origin is not merely a descriptive label but a critical determinant of quality, efficacy, and safety. Thorough understanding of the plant’s taxonomy, cultivation practices, and sourcing location is essential for manufacturers, healthcare professionals, and consumers to make informed decisions. Challenges in botanical authentication and quality control require ongoing research and stringent regulatory oversight to ensure the integrity of plant-derived wellness products.
2. Active compounds
The efficacy and safety of plant-derived wellness aids are intrinsically linked to their active compounds. These bioactive constituents are responsible for the purported pharmacological effects. A comprehensive understanding of these compounds is crucial for assessing potential benefits and risks.
- Identification and Characterization
The first step in understanding the activity of these products is the precise identification and characterization of its active compounds. Techniques such as chromatography and mass spectrometry are employed to isolate and identify these constituents. For example, the identification of curcuminoids as the primary active compounds in turmeric allows researchers to study their specific effects on inflammation.
- Mechanism of Action
Once identified, the mechanism of action of the active compounds must be elucidated. This involves understanding how these compounds interact with biological systems at the molecular level. For instance, the hypoglycemic effect of berberine, found in some plant species, is attributed to its impact on glucose metabolism pathways. A thorough understanding of the mechanisms is critical for evaluating their therapeutic potential and predicting potential side effects.
- Bioavailability and Metabolism
The bioavailability and metabolism of active compounds significantly impact their efficacy. Bioavailability refers to the extent to which a compound is absorbed into the bloodstream and made available at its site of action. Metabolism describes the process by which the body breaks down and eliminates these compounds. Factors influencing bioavailability and metabolism include the chemical structure of the compound, the route of administration, and individual variations in metabolism. For example, the low bioavailability of curcumin has led to the development of formulations that enhance its absorption.
- Standardization and Quality Control
Standardization and quality control measures are essential to ensure the consistent presence of active compounds in plant-derived products. Standardization involves setting specific limits for the concentration of key active compounds. Quality control measures, such as testing for purity and potency, are implemented to verify that products meet these standards. This ensures that consumers receive products with consistent and predictable effects. For example, Ginseng products are often standardized to contain a certain percentage of ginsenosides.
The study of active compounds is fundamental to understanding the effects of plant-derived wellness aids. The interplay between identification, mechanism of action, bioavailability, and standardization significantly influences the safety and efficacy of these products. Further investigation and rigorous quality control are vital for ensuring consistent and reliable benefits to consumers.
3. Traditional uses
The historical employment of plants in traditional medicine forms a foundational element in the development and understanding of many plant-derived wellness aids. These practices, often rooted in centuries of observation and empirical knowledge, provide insights into potential therapeutic applications. The cause-and-effect relationships observed over generations by traditional healers have guided the selection and application of botanicals for various health conditions. The documentation of these uses, passed down through oral tradition and written texts, represents a valuable resource for modern researchers seeking to validate efficacy and identify active compounds. For example, the traditional use of willow bark to alleviate pain predates the discovery of salicylic acid, the active ingredient in aspirin.
The importance of traditional uses lies in their ability to provide initial clues regarding the potential benefits of specific plants. While not a substitute for rigorous scientific investigation, ethnobotanical data can direct research efforts towards compounds with a higher likelihood of possessing therapeutic activity. Furthermore, traditional knowledge can inform the optimal methods of preparation, dosage, and administration. The Ayurvedic system of medicine, for instance, employs complex formulations and processing techniques to enhance the bioavailability and efficacy of herbal remedies. Understanding these traditional practices is essential for translating historical knowledge into evidence-based applications.
However, it is crucial to acknowledge the limitations of relying solely on traditional uses. The absence of standardized methodologies and controlled trials in traditional practices necessitates critical evaluation and scientific validation. Contemporary research aims to isolate active compounds, elucidate mechanisms of action, and conduct clinical trials to confirm efficacy and safety. Integrating traditional knowledge with modern scientific methods allows for a more comprehensive understanding of the potential benefits and risks associated with plant-derived wellness aids, ensuring responsible and informed use.
4. Dosage regulation
Dosage regulation is a critical determinant of safety and efficacy in the context of plant-derived wellness aids. Unlike conventional pharmaceuticals, the concentration of active compounds in these products can vary significantly due to factors such as plant variety, growing conditions, and processing methods. Consequently, the lack of precise dosage control poses inherent challenges. Inadequate dosage may render a product ineffective, while excessive dosage can lead to adverse effects, including toxicity. For example, excessive consumption of certain herbal teas containing senna can result in severe gastrointestinal distress and electrolyte imbalances.
The absence of stringent regulatory standards for plant-derived products in many jurisdictions further complicates dosage regulation. Manufacturers may not be required to conduct rigorous testing to determine the exact quantity of active compounds in each dose. This lack of transparency necessitates caution on the part of consumers and healthcare professionals. Standardization of herbal extracts to contain specific concentrations of marker compounds (e.g., ginsenosides in ginseng) represents one approach to improve dosage control. However, even standardized extracts may exhibit batch-to-batch variability. Consulting with a knowledgeable healthcare provider, particularly one with expertise in botanical medicine, is advisable to determine appropriate dosage and monitor for potential adverse effects.
In summary, dosage regulation is paramount for ensuring the safe and effective use of plant-derived wellness aids. Variability in product composition and regulatory oversight underscores the need for caution and professional guidance. Standardization of extracts, although beneficial, does not eliminate the inherent challenges associated with dosage control. Continued research and enhanced regulatory standards are essential to improve the consistency and reliability of these products.
5. Potential interactions
The use of plant-derived wellness aids necessitates a careful consideration of potential interactions with prescription medications, over-the-counter drugs, and other botanical products. These interactions can significantly alter the therapeutic effects and safety profiles of both the botanical product and the co-administered substance.
- Pharmacokinetic Interactions
Pharmacokinetic interactions occur when plant-derived compounds affect the absorption, distribution, metabolism, or excretion of a medication. For instance, St. John’s Wort, a commonly used botanical for mood support, is known to induce CYP3A4 enzymes, which are responsible for metabolizing numerous medications, including certain antidepressants, oral contraceptives, and statins. This induction can lead to decreased levels of these medications in the bloodstream, potentially reducing their efficacy. Conversely, other plant-derived substances may inhibit these enzymes, increasing drug levels and the risk of toxicity. Grapefruit juice, while technically a food product, contains compounds that inhibit CYP3A4, leading to increased levels of certain statins and anti-anxiety medications.
- Pharmacodynamic Interactions
Pharmacodynamic interactions arise when plant-derived compounds and medications have additive or antagonistic effects on the same physiological system. For example, combining ginkgo biloba, which has antiplatelet effects, with anticoagulant medications such as warfarin or aspirin, can increase the risk of bleeding. Similarly, the concomitant use of plant-derived products with sedative properties, such as valerian or chamomile, alongside prescription sedatives or alcohol can potentiate central nervous system depression, leading to drowsiness, impaired coordination, and respiratory suppression.
- Herb-Drug Interactions in Specific Populations
Certain populations are particularly vulnerable to herb-drug interactions. Older adults, who often take multiple medications for chronic conditions, are at increased risk due to age-related changes in drug metabolism and excretion. Pregnant and breastfeeding women require special consideration, as some plant-derived compounds can cross the placenta or be excreted into breast milk, potentially affecting the fetus or infant. Children are also more susceptible to adverse effects due to their immature metabolic pathways. Therefore, the use of plant-derived products in these populations warrants close monitoring and professional guidance.
- Lack of Standardization and Information
The lack of standardization in plant-derived wellness aids and the limited availability of comprehensive interaction data pose significant challenges. Many products lack standardized dosages of active compounds, making it difficult to predict the magnitude of potential interactions. Furthermore, the available scientific literature on herb-drug interactions is often incomplete or contradictory. This underscores the need for healthcare providers to take a thorough medication history, including the use of plant-derived products, and to exercise caution when recommending or co-administering these substances.
The potential for interactions between plant-derived wellness aids and conventional medications is a complex and clinically relevant issue. A thorough understanding of pharmacokinetic and pharmacodynamic mechanisms, consideration of vulnerable populations, and awareness of the limitations of available information are essential for mitigating risks and ensuring patient safety. Healthcare providers play a crucial role in educating patients about potential interactions and promoting informed decision-making regarding the use of these products.
6. Quality control
In the context of plant-derived wellness products, quality control is not merely a procedural formality but a critical safeguard for public health and consumer confidence. Given the inherent variability in plant material and the complexity of extraction processes, robust quality control measures are essential to ensure product safety, efficacy, and consistency.
- Botanical Authentication
Accurate identification of plant species is the first and foremost step in quality control. DNA barcoding, microscopy, and macroscopic examination are employed to verify the botanical origin of raw materials. Misidentification or adulteration with related species can lead to ineffective or even harmful products. For example, the substitution of Aristolochia fangchi for Stephania tetrandra in weight loss products has resulted in kidney failure due to the presence of aristolochic acid.
- Purity Testing
Plant-derived products are susceptible to contamination with heavy metals, pesticides, microbial pathogens, and other adulterants. Rigorous testing for these contaminants is crucial to ensure product safety. Inductively coupled plasma mass spectrometry (ICP-MS) is used to quantify heavy metal levels, while gas chromatography-mass spectrometry (GC-MS) is employed to detect pesticide residues. Microbial testing involves culturing and identifying potential pathogens such as Salmonella and E. coli.
- Active Compound Standardization
To ensure consistent efficacy, plant-derived extracts are often standardized to contain specific concentrations of key active compounds. High-performance liquid chromatography (HPLC) is commonly used to quantify these compounds. For instance, Ginkgo biloba extracts are standardized to contain a defined percentage of flavone glycosides and terpene lactones. Standardization aims to minimize batch-to-batch variability and provide consumers with a reliable product.
- Stability Testing
Plant-derived products can degrade over time, leading to a loss of potency and the formation of degradation products. Stability testing involves storing products under controlled conditions of temperature and humidity and periodically analyzing them to assess their chemical and physical stability. This testing helps to determine the shelf life of the product and ensure that it remains effective until its expiration date.
The facets of quality control highlighted above collectively contribute to ensuring that plant-derived wellness products meet established standards of safety, efficacy, and consistency. The application of sophisticated analytical techniques and adherence to Good Manufacturing Practices (GMP) are essential for maintaining product integrity and safeguarding public health. Furthermore, ongoing research and development of improved quality control methodologies are crucial to address the evolving challenges in this field.
7. Scientific evidence
The evaluation of plant-derived wellness aids necessitates a rigorous assessment of scientific evidence. The efficacy and safety of these products must be substantiated through well-designed clinical trials and preclinical studies. Reliance solely on traditional uses or anecdotal evidence is insufficient to establish therapeutic value or identify potential risks. Scientific evidence serves as the cornerstone for informed decision-making by healthcare professionals and consumers.
- Clinical Trial Rigor
Clinical trials are essential for determining the efficacy of plant-derived products in human populations. The strength of the evidence is dependent on the trial design, sample size, and methodology. Randomized, double-blind, placebo-controlled trials are considered the gold standard. These studies minimize bias and allow for a clear assessment of cause-and-effect relationships. For example, a well-designed clinical trial demonstrating a statistically significant reduction in blood pressure with hibiscus extract provides stronger evidence than observational studies.
- Preclinical Studies and Mechanisms of Action
Preclinical studies, including in vitro and in vivo experiments, are crucial for elucidating the mechanisms of action of plant-derived compounds. These studies can identify potential therapeutic targets and predict pharmacological effects. For example, preclinical research demonstrating that curcumin inhibits inflammatory pathways supports its potential use in treating inflammatory conditions. However, it is important to note that findings from preclinical studies must be confirmed in human clinical trials.
- Systematic Reviews and Meta-Analyses
Systematic reviews and meta-analyses provide a comprehensive overview of the available scientific evidence on a particular plant-derived product. These studies synthesize data from multiple clinical trials to provide a more precise estimate of the treatment effect. A meta-analysis of clinical trials evaluating the efficacy of St. John’s Wort for depression, for instance, can provide a more robust assessment of its effectiveness than any single trial alone.
- Quality and Consistency of Evidence
The quality and consistency of the scientific evidence must be critically evaluated. Factors such as methodological flaws, publication bias, and heterogeneity of study populations can influence the reliability of the findings. The GRADE (Grading of Recommendations Assessment, Development and Evaluation) system is often used to assess the quality of evidence and the strength of recommendations. A high-quality systematic review with consistent findings across multiple trials provides stronger evidence than a single, poorly designed study.
In summary, the evaluation of plant-derived wellness aids requires a thorough assessment of scientific evidence, including clinical trial rigor, preclinical studies, systematic reviews, and the quality of the evidence. Scientific evidence informs the rational use of these products, promotes patient safety, and guides healthcare decision-making. The continuous advancement of scientific research in this field is essential for providing evidence-based recommendations.
Frequently Asked Questions About Herbal Supplements
This section addresses common inquiries regarding plant-derived wellness aids, offering objective information to promote informed decision-making.
Question 1: Are plant-derived wellness aids inherently safe because they are “natural”?
The term “natural” does not equate to safety. Plant-derived products contain bioactive compounds that can exert potent pharmacological effects. Like conventional medications, these products can cause adverse reactions, interact with other substances, and may be contraindicated in certain individuals or conditions. Thorough research and professional guidance are essential.
Question 2: Are plant-derived wellness aids regulated as strictly as prescription medications?
The regulatory landscape for plant-derived products varies significantly across jurisdictions. In many regions, these products are subject to less stringent regulations than prescription medications. This may result in variations in product quality, standardization, and labeling accuracy. Consumers must exercise caution and choose reputable brands.
Question 3: Can plant-derived wellness aids be used as a substitute for conventional medical treatment?
Plant-derived products should not be used as a substitute for conventional medical treatment without consulting a qualified healthcare provider. While some may offer complementary benefits, they are not a replacement for evidence-based medical interventions for serious health conditions. Delaying or foregoing conventional treatment in favor of plant-derived products can have serious consequences.
Question 4: How can the quality of plant-derived wellness aids be assessed?
Assessing product quality involves several factors. Look for products from reputable manufacturers that adhere to Good Manufacturing Practices (GMP). Check for third-party certifications, such as those from NSF International or USP, which indicate independent testing for purity and potency. Scrutinize ingredient lists and avoid products with vague or unsubstantiated claims.
Question 5: What are the potential risks associated with plant-derived wellness aids?
Potential risks include adverse reactions (e.g., allergic reactions, gastrointestinal upset), interactions with medications or other supplements, contamination with heavy metals or pesticides, and variability in product potency. Individuals with pre-existing medical conditions, pregnant or breastfeeding women, and children should exercise particular caution and seek professional guidance.
Question 6: Where can reliable information about plant-derived wellness aids be found?
Reliable information can be obtained from reputable sources such as peer-reviewed scientific literature, government health agencies (e.g., National Institutes of Health), and professional organizations specializing in botanical medicine. Be wary of information from websites with biased or unsubstantiated claims.
In conclusion, plant-derived wellness aids represent a complex category of products with potential benefits and risks. A cautious and informed approach, guided by scientific evidence and professional advice, is essential for responsible use.
The next section will summarize key considerations and offer final thoughts regarding plant-derived wellness aids.
Conclusion
This exploration of herbal supplements reveals a complex landscape demanding informed navigation. Key considerations include botanical origins, active compounds, traditional uses, dosage regulation, potential interactions, quality control, and the strength of scientific evidence. A superficial understanding of these elements can lead to ineffective or even harmful outcomes. Rigorous scrutiny and cautious application are therefore paramount.
The responsible use of herbal supplements requires a commitment to ongoing education and a willingness to engage with qualified healthcare professionals. Further research and enhanced regulatory oversight are essential to ensure the safety, efficacy, and consistency of these products. While the allure of plant-derived remedies persists, a discerning approach founded on scientific principles remains the most prudent path forward.




![What is Herbal Preparations Definition? [Guide] The Ultimate Herbal Remedies Guide: Natural Healing for a Healthier Life What is Herbal Preparations Definition? [Guide] | The Ultimate Herbal Remedies Guide: Natural Healing for a Healthier Life](https://umangherbals.com/wp-content/uploads/2026/02/th-201-300x200.jpg)