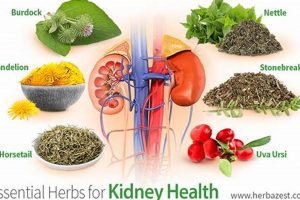
Botanical preparations intended for consumption or topical application represent a significant category within health and wellness. These items, derived from plants, often contain a complex mixture of bioactive compounds. An example includes supplements made from echinacea, purported to support immune function, or topical ointments using calendula for skin soothing properties.
The appeal of these offerings stems from perceived natural origins and, in some cases, historical use in traditional medicinal systems. Benefits are often attributed to synergistic effects of multiple plant constituents, although rigorous scientific validation may vary. Historically, many cultures have relied on these plant-based remedies for addressing various health concerns, establishing a long-standing relationship between humans and the botanical world.
Understanding the composition, regulatory landscape, and appropriate application of plant-derived wellness solutions is paramount. Subsequent sections will explore specific categories, potential interactions, and considerations for responsible use, providing a detailed analysis of this broad field.
Guidance on Botanical Preparations
The following guidance offers practical advice for informed decision-making regarding plant-derived wellness solutions. It emphasizes responsible usage and a critical evaluation of available information.
Tip 1: Prioritize Source Transparency: Scrutinize the origin and processing methods. Opt for manufacturers that provide detailed information about sourcing, extraction, and quality control procedures. Look for certifications verifying adherence to specific standards.
Tip 2: Investigate Bioactive Compounds: Understand the key components and their potential effects. Research the plant’s known chemical constituents and their established physiological actions. Consult reputable scientific databases for relevant studies.
Tip 3: Consult with Healthcare Professionals: Seek advice from qualified medical practitioners. Discuss potential interactions with existing medications, pre-existing conditions, or individual health considerations. Do not self-diagnose or replace conventional medical treatments.
Tip 4: Adhere to Recommended Dosages: Strictly follow dosage instructions provided by the manufacturer or healthcare provider. Overconsumption may lead to adverse effects or diminished efficacy. Underdosing may negate intended benefits.
Tip 5: Monitor for Adverse Reactions: Pay close attention to any unusual symptoms or side effects. Discontinue use immediately if adverse reactions occur, and seek medical attention if necessary. Allergic reactions, gastrointestinal distress, and skin irritations are potential concerns.
Tip 6: Verify Regulatory Compliance: Confirm that the product adheres to relevant regulatory standards in the jurisdiction of sale. Regulations may vary significantly across different regions. Check for appropriate labeling and certifications.
Tip 7: Exercise Caution with Unsubstantiated Claims: Be wary of exaggerated or unsubstantiated health claims. Scientifically valid evidence should support therapeutic claims. Critically evaluate marketing materials and rely on reputable sources for information.
By adhering to these guidelines, individuals can approach botanical preparations with greater awareness and minimize potential risks. Informed choices contribute to responsible use and maximize the potential benefits of these natural substances.
The following sections will delve deeper into the current regulatory landscape and offer a comparative analysis of various preparation types.
1. Botanical Source Identification
Botanical Source Identification serves as a fundamental pillar in the responsible production and use of plant-derived wellness solutions. The precise botanical origin of a plant preparation directly influences its chemical composition and, consequently, its potential therapeutic effects. Mislabelling or inaccurate identification can lead to inconsistent product quality, unexpected side effects, or even outright ineffectiveness. For instance, using the wrong species of ginseng (e.g., American ginseng instead of Asian ginseng) results in different ginsenoside profiles, thereby altering the expected physiological impact. The effect is direct and significant.
The importance of accurate identification extends beyond simply knowing the correct genus and species. Factors such as the plant’s chemotype (chemical variant within a species), geographic origin, and cultivation practices can also influence its bioactive constituents. An example is found in Hypericum perforatum (St. John’s Wort), where the concentration of hypericin, a key antidepressant compound, varies widely depending on where and how the plant is grown. Therefore, robust analytical methods, including macroscopic and microscopic examination, as well as DNA barcoding, are crucial for authenticating botanical sources and ensuring consistent quality in plant preparations.
In conclusion, the effective utilization of botanical preparations relies heavily on accurate Botanical Source Identification. This process is not merely a perfunctory step, but rather a critical control point that directly affects product safety, efficacy, and overall quality. Accurate identification mitigates the risk of adulteration, substitution, and inconsistent performance, fostering greater confidence in these types of preparations. Addressing the challenges associated with botanical authentication is paramount to ensuring the responsible and sustainable use of plant-derived resources.
2. Bioactive Compound Analysis
Bioactive Compound Analysis forms a cornerstone in the understanding and utilization of plant-derived products. It is the process of identifying and quantifying the chemical constituents within a preparation that are responsible for its purported effects. The presence and concentration of these compounds directly influence the product’s efficacy and safety profile. Without proper analysis, the user is essentially consuming or applying an unknown mixture, potentially leading to unpredictable results or adverse reactions. A real-world example lies in the standardization of milk thistle extract based on its silymarin content. This ensures that each dose delivers a consistent amount of the liver-protective compounds.
The analysis provides a means to ensure batch-to-batch consistency, critical for maintaining predictable therapeutic outcomes. Techniques like chromatography and mass spectrometry are employed to meticulously characterize the chemical composition. Beyond quantification, understanding the synergistic or antagonistic interactions between different bioactive components is crucial. For instance, certain compounds may enhance the absorption or activity of others, while others may inhibit them. This knowledge can inform formulation strategies to optimize the product’s overall effect. A practical application of Bioactive Compound Analysis is in the development of quality control standards for herbal ingredients, ensuring that only products meeting specific compositional criteria reach the market.
In summary, Bioactive Compound Analysis is indispensable for ensuring the quality, safety, and efficacy of botanical preparations. It moves beyond anecdotal evidence by providing a scientific basis for understanding and predicting product performance. Addressing the inherent variability in plant chemistry through rigorous analysis is essential for promoting responsible and informed usage. The challenges lie in the complexity of plant matrices and the need for advanced analytical techniques, highlighting the importance of investing in robust research and quality control measures. This is essential for product herbal to be safe.
3. Standardized Extraction Methods
Standardized Extraction Methods exert a direct influence on the composition and quality of a botanical preparation. The extraction process determines which compounds are isolated from the plant material and, consequently, the product’s therapeutic potential. Variations in extraction parameters, such as solvent, temperature, and duration, can significantly alter the resulting extract’s chemical profile. For instance, using water as a solvent may yield a different set of compounds compared to using ethanol, impacting the final product. Therefore, employing standardized methods is crucial for achieving consistency and reproducibility in plant-derived product manufacturing. This consistency reduces variations in the chemical profile of the finished good, ultimately giving a consumer a product that more closely matches the intended benefits.
The significance of standardized extraction becomes apparent when considering the challenges of working with complex natural matrices. Plant materials inherently exhibit variability due to factors such as geographic location, growing conditions, and harvest time. Standardized extraction methods help to minimize this variability by controlling the isolation of specific compounds. A practical application involves the extraction of curcuminoids from turmeric. By standardizing the extraction process, manufacturers can ensure a consistent concentration of these bioactive compounds, enabling accurate dosage and predictable therapeutic outcomes. The consequences of non-standardized extraction may lead to inconsistent quality, reduced efficacy, or even potential safety concerns, depending on the final concentrations of chemicals in the product.
In summary, Standardized Extraction Methods are indispensable for ensuring the quality, efficacy, and safety of botanical preparations. These methods are implemented in response to inherent variability in the plant sources. Consistent extraction is essential for product herbal success. Without the standardization of these methods, it would be near impossible to offer any consistency in finished good quality to customers. Ultimately, a commitment to these principles fosters greater confidence and trust in the benefits associated with product herbal.
4. Dosage Recommendation Adherence
Dosage Recommendation Adherence is a critical factor influencing the safety and efficacy of plant-derived preparations. Deviation from recommended dosages can result in either a lack of therapeutic effect or, conversely, the manifestation of adverse reactions. The inherent complexity of botanical constituents necessitates a careful approach to dosage, as individual responses can vary based on factors such as age, body weight, and pre-existing health conditions.
- Safety Profile Maintenance
Adhering to recommended dosages is essential for minimizing the risk of adverse effects associated with plant preparations. Exceeding the recommended dose can lead to toxicities or undesirable side effects, such as gastrointestinal distress, allergic reactions, or interactions with conventional medications. For example, excessive consumption of licorice root can result in elevated blood pressure and electrolyte imbalances. Conversely, insufficient dosage may fail to elicit the intended therapeutic response, leading to patient disappointment and potentially delaying appropriate medical intervention.
- Efficacy Optimization
The effectiveness of many plant preparations is dose-dependent, meaning that the therapeutic benefit is only realized within a specific dosage range. Underdosing may result in a lack of noticeable improvement, while overdosing may not necessarily enhance efficacy and could increase the risk of adverse effects. Controlled clinical trials are often used to establish optimal dosage regimens, and adherence to these recommendations is crucial for maximizing the potential benefits. An example lies in the use of St. John’s Wort for mild to moderate depression, where specific doses have been shown to be more effective than others.
- Consistency in Bioavailability
Dosage recommendations often take into account factors affecting the bioavailability of active compounds. Bioavailability refers to the extent and rate at which a substance is absorbed into the bloodstream. Some compounds may have low bioavailability, requiring higher doses for a therapeutic effect. Adherence to recommended dosages ensures that the body receives a sufficient amount of the active compound to exert its intended effect. Formulation strategies, such as encapsulation or liposomal delivery, may be employed to enhance bioavailability, but the prescribed dosage remains paramount. An example is seen with curcumin, where co-administration with piperine (found in black pepper) enhances its absorption.
- Patient-Specific Considerations
While general dosage recommendations provide a starting point, individual patient characteristics may necessitate adjustments. Factors such as age, body weight, liver and kidney function, and concurrent medication use can influence the metabolism and elimination of plant-derived compounds. Consultation with a healthcare professional is crucial for determining the appropriate dosage for specific individuals, particularly those with underlying health conditions or those taking multiple medications. For instance, individuals with impaired kidney function may require lower doses of certain plant preparations to avoid accumulation and potential toxicity.
Dosage Recommendation Adherence, therefore, represents a critical interface between the inherent properties of botanical preparations and the individual needs of the consumer. A conscientious approach to dosage is paramount for maximizing the benefits of product herbal while minimizing the risks associated with their use.
5. Regulatory Compliance Verification
Regulatory Compliance Verification is a pivotal aspect of ensuring the safety, quality, and legality of plant-derived preparations. The market for items derived from botanicals operates within a complex framework of national and international regulations designed to protect consumers from harmful or ineffective products. Verification processes confirm that a particular product adheres to these established standards, encompassing aspects such as manufacturing practices, labeling requirements, and ingredient safety. For instance, a product labelled as “organic” must undergo certification by an accredited body, verifying adherence to specific cultivation and processing standards. Without this, a product herbal can be untrustworthy.
The consequences of non-compliance can be far-reaching, ranging from product recalls and fines to reputational damage and, most importantly, potential harm to consumers. For example, the presence of undeclared allergens or contaminants in a botanical supplement can trigger severe allergic reactions in susceptible individuals. Moreover, misleading or unsubstantiated health claims can deceive consumers into using these products inappropriately or delaying necessary medical treatment. Regulatory agencies, such as the FDA in the United States or the EMA in Europe, play a crucial role in enforcing compliance and safeguarding public health. Product herbal sellers must also be knowledgable of all legal regulations.
In conclusion, Regulatory Compliance Verification serves as a cornerstone of responsible plant-derived product manufacturing and marketing. Its importance cannot be overstated, as it directly impacts consumer safety, product quality, and overall market integrity. While navigating the complex regulatory landscape can be challenging, adherence to established standards is essential for ensuring the legitimacy and trustworthiness of product herbal. It is a collective responsibility shared by manufacturers, regulators, and consumers alike to uphold these standards and promote the safe and effective use of plant-derived remedies.
6. Potential Interaction Awareness
Potential Interaction Awareness is a critical component in the responsible use of plant-derived preparations, as many “product herbal” can interact with conventional medications, other supplements, or even certain foods. These interactions can alter the intended effects of either the medication or the botanical, potentially leading to reduced efficacy or increased toxicity. The complex chemical composition of plant materials is the primary cause of such interactions. For example, St. John’s Wort, commonly used for mild depression, can significantly reduce the effectiveness of certain medications, including oral contraceptives and immunosuppressants, due to its impact on liver enzymes. This exemplifies why recognizing the potential for interactions is crucial to avoid adverse consequences, or in the least, mitigate these.
Understanding pharmacokinetic and pharmacodynamic interactions is essential for healthcare professionals and consumers alike. Pharmacokinetic interactions affect the absorption, distribution, metabolism, and excretion of drugs, while pharmacodynamic interactions involve the combined effects of drugs on the body. For instance, taking garlic supplements concurrently with anticoagulant medications, such as warfarin, can increase the risk of bleeding due to garlic’s inherent antiplatelet properties. Similarly, grapefruit juice, known to inhibit certain liver enzymes, can alter the metabolism of numerous medications, potentially leading to elevated drug levels and increased side effects. The significance of this understanding translates into the ability to make informed decisions about combining “product herbal” with other treatments, minimizing risks, and optimizing therapeutic outcomes.
In summary, Potential Interaction Awareness forms an integral part of the safe and effective use of “product herbal”. Failure to consider these interactions can have serious health consequences. While botanical remedies may offer potential benefits, they are not without risks, and a comprehensive understanding of their potential interactions is paramount for promoting responsible self-care and preventing adverse outcomes. Challenges persist in fully elucidating all possible interactions due to the vast number of plant species and chemical constituents involved. This underscores the need for ongoing research, improved labeling practices, and enhanced communication between healthcare professionals and consumers.
7. Efficacy Validation Scrutiny
Efficacy Validation Scrutiny represents a critical process in determining the therapeutic value of botanical preparations. Due to the complex chemical composition of plant-derived substances and the variability inherent in natural products, rigorous scientific evaluation is essential to substantiate any claimed benefits. This scrutiny ensures that consumers are not misled by unsubstantiated claims and that healthcare professionals have access to reliable information for informed decision-making.
- Randomized Controlled Trials (RCTs)
RCTs form the gold standard for assessing the efficacy of medical interventions, including botanical preparations. These trials involve randomly assigning participants to either a treatment group receiving the botanical product or a control group receiving a placebo. By comparing outcomes between the two groups, researchers can determine whether the botanical product has a statistically significant effect. For example, RCTs investigating the efficacy of Echinacea for the common cold have yielded mixed results, highlighting the need for careful study design and analysis. Positive trials showcase an ability to reduce cold duration, while some show no significance over placebo.
- Systematic Reviews and Meta-Analyses
Systematic reviews and meta-analyses provide a comprehensive overview of existing research on a particular topic. These studies systematically identify, evaluate, and synthesize the results of multiple RCTs to determine the overall effectiveness of a botanical preparation. Meta-analyses can increase statistical power and provide more precise estimates of treatment effects. The Cochrane Library, for example, contains numerous systematic reviews evaluating the efficacy of various herbal remedies for different health conditions, helping to give insight to scientific consensus.
- Dose-Response Studies
Dose-response studies are essential for determining the optimal dosage of a botanical preparation. These studies evaluate the effects of different doses on clinical outcomes to identify the dosage range that provides the maximum benefit with minimal side effects. For instance, dose-response studies have been conducted to determine the optimal dosage of Ginkgo biloba for cognitive enhancement, leading to the determination of effective dosage parameters and regimens, while avoiding dosages that could prove dangerous. Without dose-response studies, there is no scientific way to determine proper consumption amount.
- Mechanism of Action Research
Understanding the mechanism of action of a botanical preparation can provide valuable insights into its therapeutic effects. This research involves investigating how the active compounds in the preparation interact with biological systems to produce a specific outcome. By elucidating the mechanism of action, researchers can gain a better understanding of the potential benefits and risks associated with the product. For example, studies have examined the mechanisms by which curcumin, a compound found in turmeric, exerts its anti-inflammatory effects, lending credibility to it’s common use, although more research is still being conducted.
Collectively, Efficacy Validation Scrutiny encompasses a range of rigorous scientific methods used to evaluate the therapeutic potential of botanical preparations. These methods include randomized controlled trials, systematic reviews, dose-response studies, and mechanism-of-action research. By subjecting botanical products to this level of scrutiny, researchers and healthcare professionals can ensure that consumers have access to safe and effective remedies. As the market for plant-derived products continues to grow, the importance of efficacy validation becomes increasingly paramount for promoting informed decision-making and safeguarding public health. Further application of these tests for product herbal is essential.
Frequently Asked Questions Regarding Botanical Preparations
The following questions address common inquiries and concerns regarding products derived from botanical sources. These answers aim to provide clear, concise information based on current scientific understanding and regulatory guidelines.
Question 1: What constitutes a “product herbal,” and how does it differ from a pharmaceutical drug?
A botanical preparation, also known as “product herbal,” is a substance derived from plants that is intended for therapeutic or health-promoting purposes. Unlike pharmaceutical drugs, which typically contain a single, purified active compound, botanical products often contain a complex mixture of bioactive constituents. Furthermore, they may not be subject to the same rigorous regulatory scrutiny as pharmaceutical drugs, depending on the specific product and jurisdiction.
Question 2: Are botanical preparations regulated for safety and efficacy?
The level of regulatory oversight for botanical preparations varies significantly depending on the country and the intended use of the product. In some jurisdictions, botanical products are regulated as dietary supplements or traditional medicines, while in others, they may be subject to stricter regulations similar to those for pharmaceutical drugs. Consumers should research the regulatory status of a botanical product in their region and look for certifications or quality seals from reputable organizations.
Question 3: How can the quality of a product herbal be assessed?
Assessing the quality of a botanical preparation requires careful consideration of several factors. These include the botanical source of the ingredients, the manufacturing processes used to extract and process the plant material, and the presence of standardized amounts of active compounds. Look for products from reputable manufacturers that provide detailed information about their sourcing and quality control procedures. Independent laboratory testing can also provide valuable information about the purity and potency of a product.
Question 4: Can botanical preparations interact with conventional medications?
Yes, many botanical preparations can interact with conventional medications, potentially altering their effects or increasing the risk of side effects. It is essential to inform a healthcare provider about all medications, supplements, and botanical products being used to avoid potentially harmful interactions. Certain botanicals, such as St. John’s Wort, are known to interact with a wide range of medications, including antidepressants, anticoagulants, and oral contraceptives.
Question 5: What are the potential risks associated with using botanical preparations?
The use of botanical preparations carries certain risks, including allergic reactions, adverse effects, and interactions with conventional medications. Some botanical products may also be contaminated with heavy metals, pesticides, or other harmful substances. Consumers should carefully research the potential risks associated with a particular product and consult with a healthcare provider before use, especially if pregnant, breastfeeding, or taking other medications.
Question 6: Where can reliable information about botanical preparations be found?
Reliable information about botanical preparations can be obtained from several sources, including reputable scientific journals, government regulatory agencies, and professional organizations dedicated to botanical medicine. Be wary of unsubstantiated claims or information from unreliable sources. Consult a qualified healthcare professional, such as a physician, pharmacist, or herbalist, for personalized advice and recommendations.
These FAQs provide a general overview of key considerations related to botanical preparations. Further research and consultation with healthcare professionals are recommended for specific health concerns.
The following section will delve into specific categories of herbal products and their applications.
Conclusion
This exploration has underscored the multifaceted nature of botanical preparations, often termed “product herbal.” Critical facets, including botanical identification, standardized extraction, dosage adherence, regulatory compliance, interaction awareness, and efficacy validation, demand meticulous attention. Neglecting these aspects compromises safety and undermines therapeutic potential.
The responsible integration of “product herbal” into healthcare necessitates ongoing research, stringent quality control, and transparent communication between manufacturers, healthcare providers, and consumers. A commitment to scientific rigor and informed decision-making will be paramount in unlocking the true benefits of plant-derived wellness solutions while mitigating inherent risks. Further advancements in analytical techniques and regulatory frameworks are essential to ensure a sustainable and trustworthy future for herbal products.



![What is Herbal Preparations Definition? [Guide] The Ultimate Herbal Remedies Guide: Natural Healing for a Healthier Life What is Herbal Preparations Definition? [Guide] | The Ultimate Herbal Remedies Guide: Natural Healing for a Healthier Life](https://umangherbals.com/wp-content/uploads/2026/02/th-201-300x200.jpg)